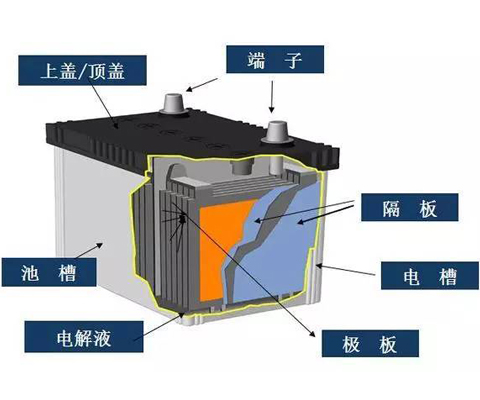

Structure of lead acid battery
Recommended reading
Structure of lead acid battery
(1) Positive plate (positive active material) The main component of the active material of the positive plate is lead dioxide, which has strong oxidizability. When discharging, it reacts with sulfuric acid to form lead sulfate, and absorbs electrons. There are two types of lattice of lead dioxide, one is lead oxide The other is PB02 PB02. These two lead dioxide active materials are very different, and they play different roles in the positive plate The capacity given by PB02 is 1.5 ~ ~ ~ 3 times of PbO2 PB02 has good mechanical strength. The active material of positive plate is not suitable to soften and fall off due to the existence of PB02 PB02 and When the ratio of PbO 2 reaches 0.8, lead-acid battery will show good performance The reaction equation is as follows: PB02 + 3H + + HSO4 + 2E = = PbSO4 + 2H2O, when charged, it is converted to lead sulfate under the action of external circuit The results s...
Contact us
No.299 Longxing West Road, Gaokai District, Baoding City, Hebei Province
+0312-5880753
+0312-5880732
E-mail: peexport@yahoo.com
Company profile
Fengfan Meixin Company integrates international advanced management concepts and systems,Passed one after another ISO9001、IATF16949、OHSAS18000 and ISO14000 Quality system certification。The company has been rated as an advanced unit of safety production...
Website : www.ffrising.com .







